General Health Tips & News
CDC Grants Approval to Novel RSV Vaccine for Infant Protection
By A.S. (staff writer) , published on August 29, 2023
Medicine Telehealth Health
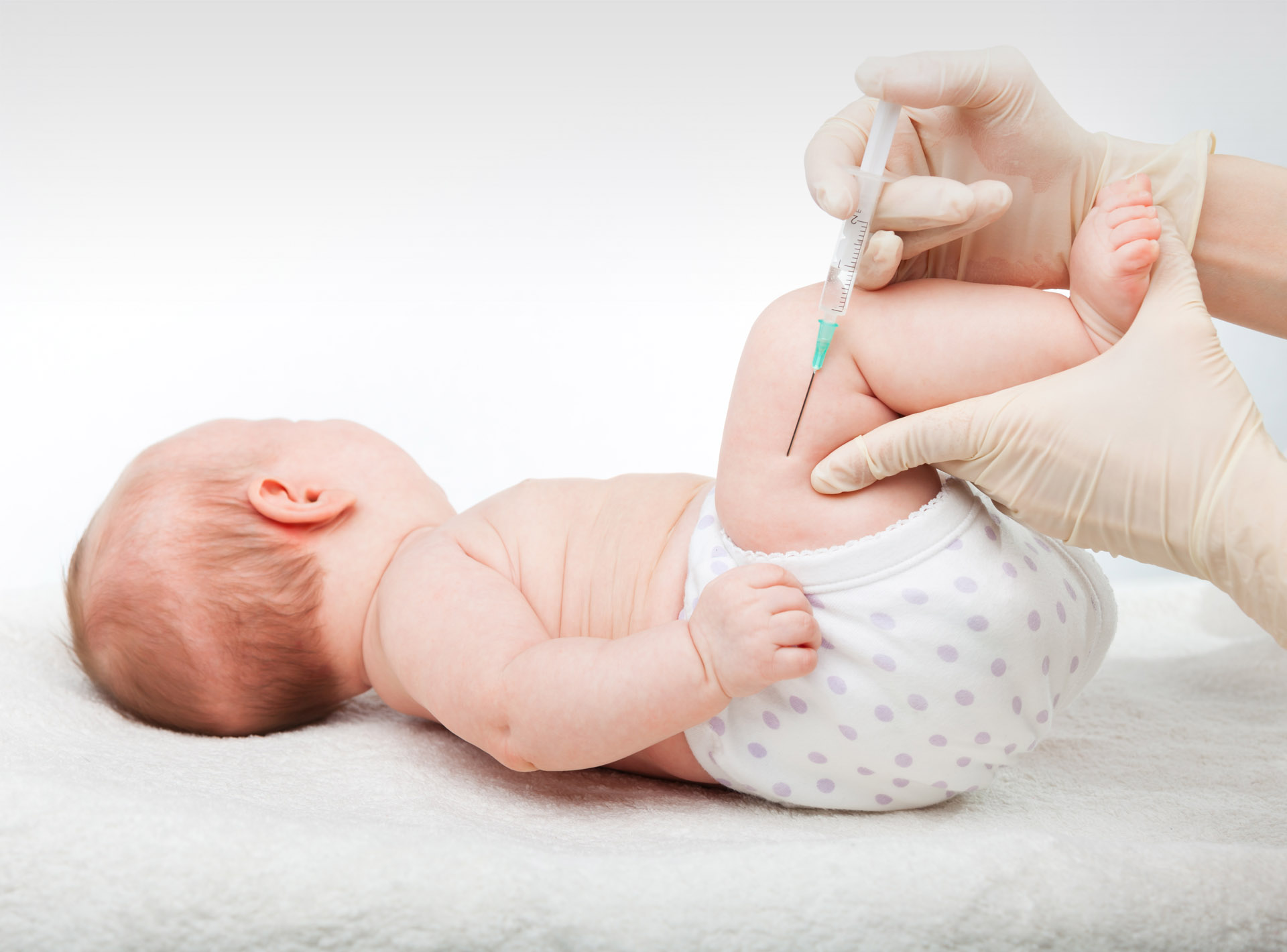
Injectable RSV Drug Recommended for Infants Under 8 Months by CDC
The Centers for Disease Control and Prevention (CDC) has made a unanimous recommendation for all babies under 8 months old, and certain older infants, to receive an injectable respiratory syncytial virus (RSV) drug starting this upcoming fall. The recommendation was endorsed by CDC Director Mandy Cohen, allowing the drug, named Beyfortus, to be distributed to the public.
In a significant move it has officially endorsed the recommendation of the CDC Advisory Committee on Immunization Practices (ACIP) regarding the utilization of Nirsevimab, also known by the trade name Beyfortus. This long-acting monoclonal antibody product has demonstrated an impressive reduction of about 80% in the risk of RSV-related hospitalizations and healthcare visits for infants.
Target Groups and Timing
The injection is aimed at two specific groups: babies up to 8 months old who are born during or entering their first RSV season (typically starting around October), and infants aged 8 to 19 months who are at higher risk of severe RSV disease and are entering their second RSV season. Newborns born just before or during the RSV season should receive the injection within their first week of life.
Mode of Action and Benefits
Beyfortus, sold as the injectable RSV drug, functions similarly to a vaccine. Instead of stimulating the immune system to create antibodies against the virus through "active immunization," it directly delivers antibodies into the bloodstream, known as "passive immunization."
Addressing RSV's Impact and Benefits for Parents
RSV can be severe in children, causing up to 80,000 hospitalizations and up to 300 deaths each year in those under the age of 5 in the U.S. The previous RSV season started early, leading to a significant rise in severe illness that overwhelmed children's hospitals.
The CDC's advisory committee member, Dr. Sarah Long, stated that this development is a breakthrough, as parents can be relieved of concerns about their children being hospitalized due to RSV disease.
Approval and Availability
The Food and Drug Administration (FDA) approved Beyfortus last month, and it is already authorized in Europe, Canada, and the United Kingdom. It is manufactured by AstraZeneca and marketed by Sanofi. Beyfortus is expected to be available in the U.S. ahead of the 2023-2024 RSV season.
Clinical Data and Side Effects
In a study involving nearly 1,500 infants, Beyfortus reduced the risk of developing an RSV-related respiratory illness requiring a doctor's visit by almost 75% for at least five months. The most common side effects observed during trials were rashes and reactions around the injection site. The FDA noted that Beyfortus could potentially trigger extreme immune responses, including anaphylaxis, which have been seen with other monoclonal antibodies.
Cost and Coverage
The anticipated cost of Beyfortus is $495, but the expense for individuals will depend on their insurance plans. Although the price is concerning to some, the CDC's Vaccines for Children Program will cover the shot, making it accessible to uninsured, underinsured, Medicare-eligible, and American Indian or Alaska Native children.
RSV and Vulnerable Populations
Most children contract RSV by age 2. While it usually causes a mild lower respiratory illness, it can lead to pneumonia or bronchiolitis in severe cases. Older adults and infants under 6 months old are particularly vulnerable to severe outcomes.
The FDA has recently approved two RSV vaccines for older adults, one from Pfizer and another from GSK. Additionally, an advisory committee recommended the approval of Pfizer's maternal RSV vaccine, administered to pregnant mothers to safeguard their infants. The FDA is expected to make a decision on this maternal vaccine next month.
References
Bendix, A. (2023, August 4). CDC recommends RSV drugs to protect babies ahead of the virus' anticipated fall spread. Retrieved from NBC News: https://www.nbcnews.com/health/health-news/cdc-advisors-recommend-rsv-shot-protect-babies-rcna97998
CDC Recommends a Powerful New Tool to Protect Infants from the Leading Cause of Hospitalization. ( 2023, August 3). Retrieved from CDC: https://www.cdc.gov/media/releases/2023/p-0803-new-tool-prevent-infant-hospitalization-.html
Find articles related to: Medicine Telehealth Health
More articles about General Health Tips & News
Back to the Health Tips Index




